 By Independent News Roundup
By Independent News Roundup
In a landmark decision, the CDC’s Advisory Committee on Immunization Practices (ACIP) voted 8–3 on Friday to eliminate the universal recommendation that all newborns receive a Hepatitis B vaccine at birth — a policy in place since 1991.

The new guidance keeps the birth dose only for infants whose mothers test positive for Hepatitis B or whose status is unknown. For all other newborns, ACIP now recommends parent-directed, individualized decision-making, with vaccination suggested no earlier than two months.
The vote followed discussion of evidence showing that U.S. newborns face extremely low risk of Hepatitis B transmission absent maternal infection, and that past declines in disease were driven largely by improved blood screening and medical practices rather than universal infant dosing.
Three dissenting members warned of potential harm, but the majority concluded that a blanket recommendation is no longer scientifically justified.
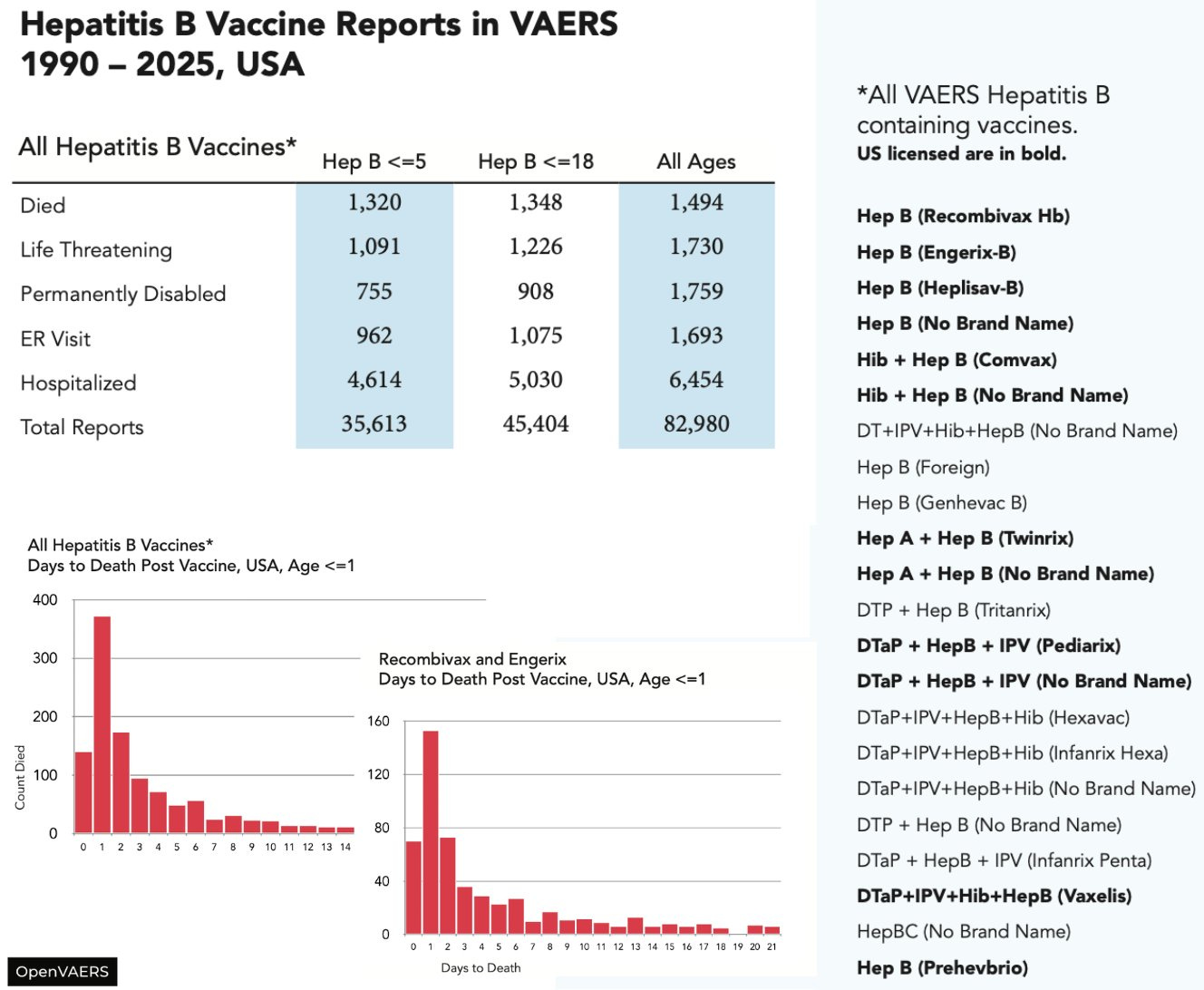
CDC VAERS data show that since 1990, mass Hepatitis B vaccination of infants and children has resulted in mass casualties:
1,494 reported deaths.
1,730 life-threatening events.
1,759 permanently disabled.
6,454 hospitalized.
82,980 adverse events.
 uTobianThere are no studies proving the safety and efficacy of hepatitis B vaccines at birth, 30 days, or 12 years of age I. Junk science clinical trials as the basis for FDA licensure of hepatitis B vaccines in the U.S…Read more3 days ago · 329 likes · 83 comments · Toby Rogers
uTobianThere are no studies proving the safety and efficacy of hepatitis B vaccines at birth, 30 days, or 12 years of age I. Junk science clinical trials as the basis for FDA licensure of hepatitis B vaccines in the U.S…Read more3 days ago · 329 likes · 83 comments · Toby RogersPending CDC director approval, this marks the first major rollback of a childhood vaccine recommendation in decades and a significant victory for medical freedom.
Epidemiologist and Foundation Administrator, McCullough Foundation
Support our mission: mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.
